The path to clinical trial & commercial success starts with connecting to patients
We power smarter trial design, recruitment, and post-market evidence with regulatory-grade data and engaged patient communities
Not your typical RWD vendor
Informed and empowered, our patient communities consent to share complete medical histories to advance better treatments
Regulatory-grade, longitudinal data
Submission-ready datasets accepted by FDA to replace traditional in-person study requirements
Comprehensive medical history
Unifies unstructured medical records, genetics, and imaging across systems from birth or diagnosis
High-density patient communities
Built with 70+ long-term advocacy partners - enabling trusted, aligned engagement
Recontactable, engaged patients
Trusted relationships enable recontact for faster recruitment and effective long-term follow-up
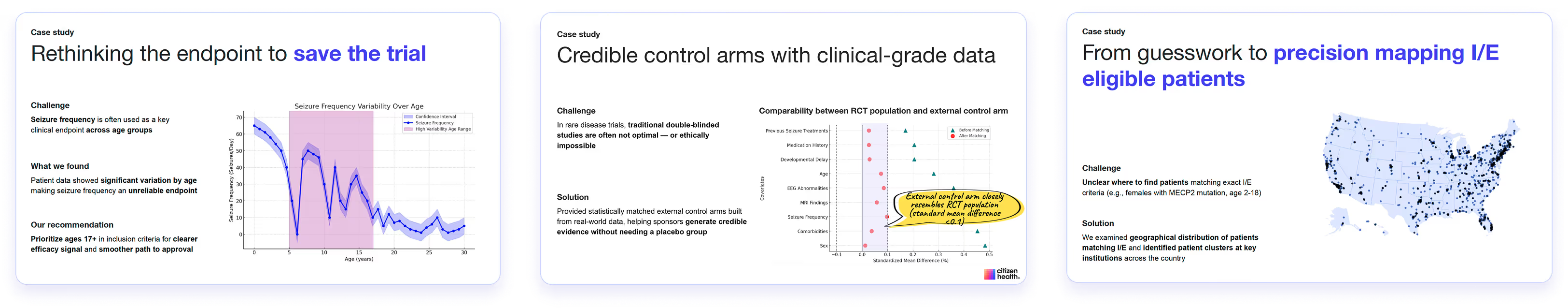
From complexity to clarity: explore our case studies
11 case studies available from pre-IND, clinical trial, to post-marketing
Have a similar challenge?
Let's talk
We are proud to partner with leading biotech and pharma companies and universities





